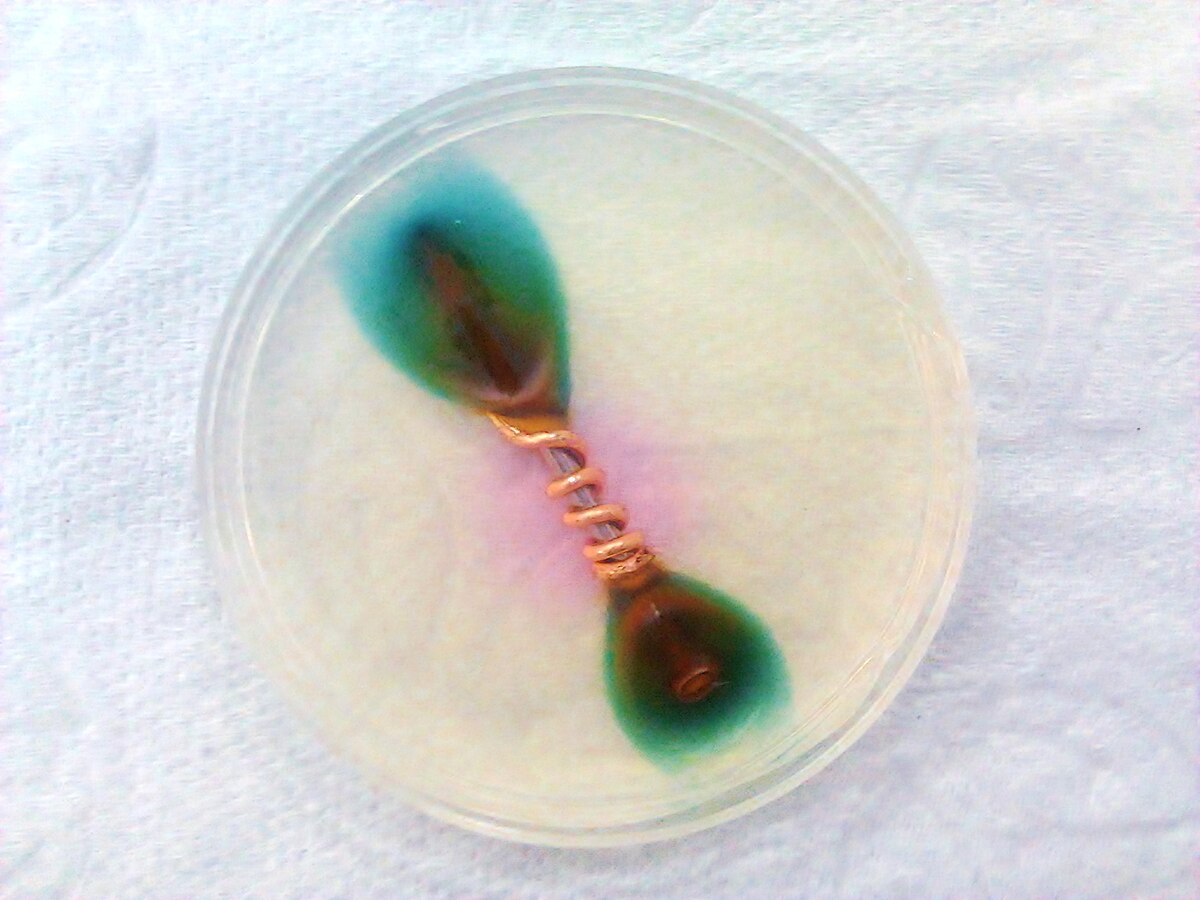
I'm trying to find out if there are any downsides to mixing metals. Specifically, I have an aluminum hawk and am wondering about using the head on a heavier handle. I was able to find some information specific to combining aluminum with stainless and there are two concerns. One is you have to be careful not to overtighten the head, stripping the screw. Second is that, over time, water + aluminum + steel = corrosion (no idea how long that would take with a razor).
Does anyone here understand metals enough to explain what would happen using an aluminum head on various metal handles? Would brass or titanium be a better choice to pair with aluminum than than steel?
Thanks!
Does anyone here understand metals enough to explain what would happen using an aluminum head on various metal handles? Would brass or titanium be a better choice to pair with aluminum than than steel?
Thanks!